Pharmaceutical Grade 1392275-56-7 Tenofovir alafenamide fumarate Pro Treatment Hepatitis Chronici B
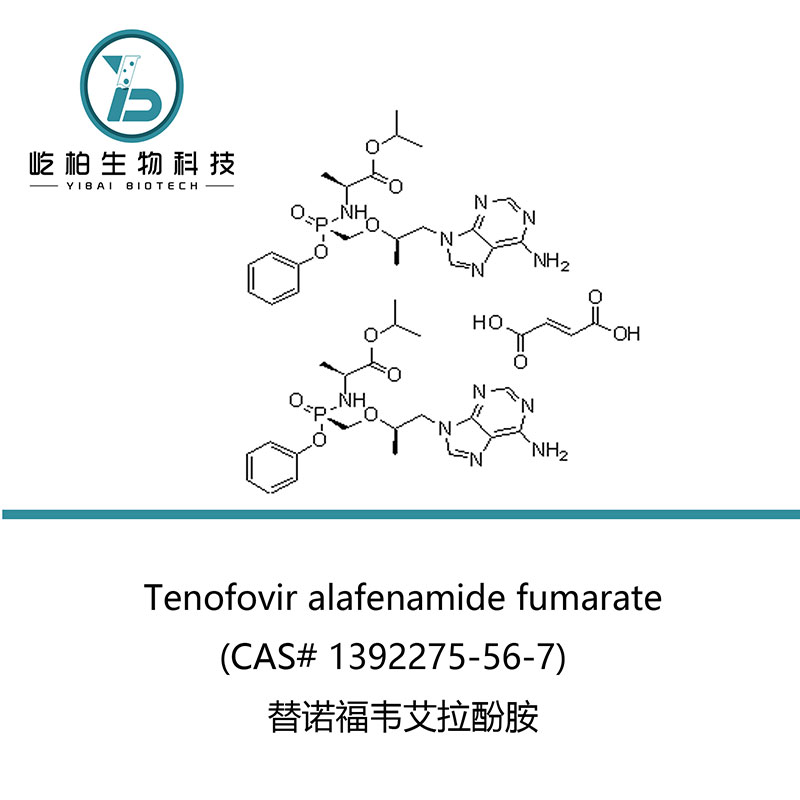
| Productumnomen | Tenofovir alafenamide fumarate |
| Synonyma | N-[(S)-[[(1R)-2-(6-Amino-9H-purin-9-yl) -1-methylethoxy]methyl]phenoxyphosphinyl]-L-alanine 1-methylethyl ester (2E)-2 -butenedioate (2, 1);GS 7340-03 |
| CAS Non. | 1392275-56-7 |
| Aspectus | Album vel paene album crystallinum pulveris |
| Formulae hypotheticae | 2(C21H29N6O5P).C*4H4O4 |
| M. Pondus | 1069.02 |
| Consuetudinem | Pharmaceutical Gradus ad Research proposito |
| stipare | Quod per petitio tua |
| Repono | Serva in stricta, levis vasis repugnans in loco frigido |
Company Information
√ Procuratio accumsan plena experientia in technicis et technicis peritissimis sectatoribus; √ Quality is always our top consideration, Strict QC system; √ 11 annos periti quadrigis venditio educendi; √ Independentes R&D lab; √ Duae officinae GMP terminus signati longi sunt; √ Divites copiarum otiosorum officinarum copia pro project nativus; √ High Efficiency working team with consistent path.


Epistulam tuam hic scribe et mitte nobis